
News Releases
Medtronic receives Health Canada licence for the Symplicity Spyral™ multi-electrode renal denervation catheter and Symplicity G3™ RF generator
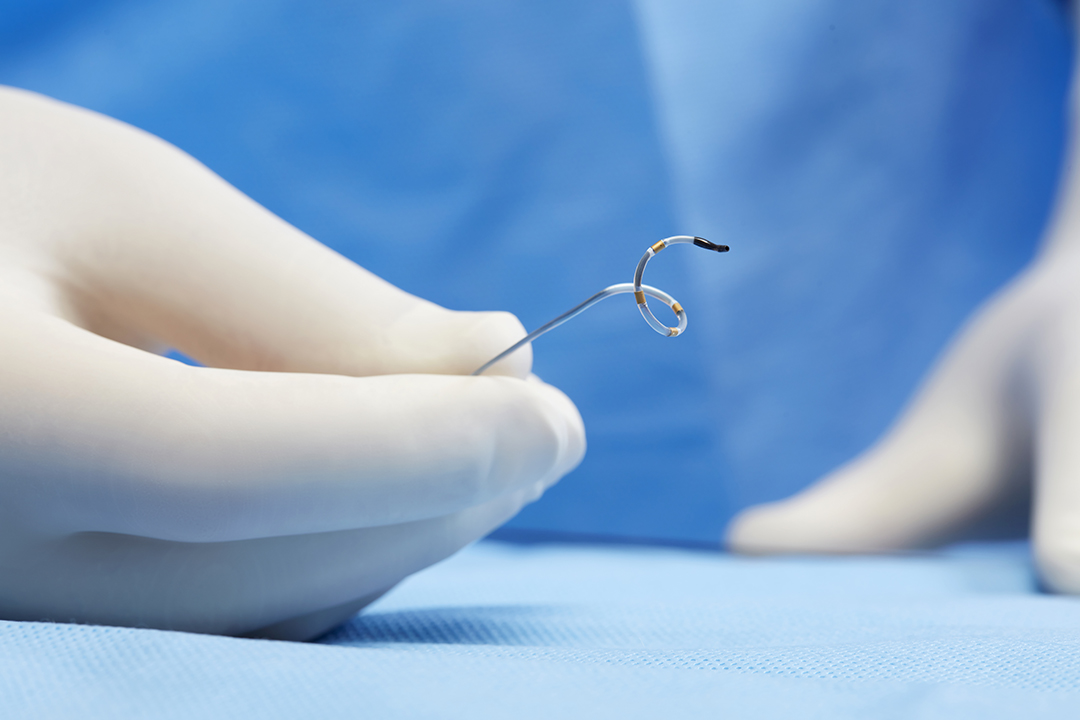
Medtronic today announced it received a license from Health Canada for its Symplicity Spyral™ multi-electrode Renal Denervation (RDN) System. This is indicated for the management of essential hypertension in patients for whom blood pressure remains uncontrolled despite lifestyle modifications and guideline-driven medical therapy with antihypertensive medications, or when guideline-driven therapy is poorly tolerated.
The Symplicity blood pressure procedure is a minimally invasive technique that uses radiofrequency ablation to calm the nerves near the kidneys that can become overactive and contribute to high blood pressure. Nearly 1 in 4 Canadian adults are affected by hypertension, and it’s estimated that 90% of individuals will develop high blood pressure at some point during their lifetime.1
“As the pioneer in renal denervation, we are looking forward to making a difference for hypertension patients in Canada,” says Maisie Cheung, director of marketing, Medtronic Canada. “This means more choices for patients — and offers physicians an adjunctive option along with lifestyle changes and medications to help manage patients with high blood pressure.”
Hypertension, or high blood pressure, is the leading modifiable cause of heart attack, stroke, and death.2 Despite available medications and lifestyle interventions, control rates remain low.3 These challenges speak to the possibility that patients may benefit from an adjunctive management option to better address their blood pressure.
“As we look to improve options to help manage patients with hypertension, we’re encouraged by this latest approval of the Symplicity Spyral renal denervation system,” said Dr. Sheldon Tobe, nephrologist and hypertension specialist, Sunnybrook Health Sciences Centre. “There is a big unmet need in Canada for the subset of patients who can’t fully control hypertension with medication and/or lifestyle changes. This innovative procedure will help to fill that gap by potentially helping to improve blood pressure control.”
Data have shown that small blood pressure reductions significantly reduce cardiovascular risk. In fact, a 5 mmHg reduction leads to a 5% lower risk of cardiovascular death, 8% lower risk of coronary artery disease, 10% lower risk of major cardiovascular events, 13% lower risk of stroke and 13% lower risk of heart failure.4
“The Symplicity blood pressure procedure opens the door to manage high blood pressure in the long term through a safe and effective procedure,” said Mina Madan, MD, interventional cardiologist, Schulich Heart Program Sunnybrook Health Sciences Centre. “It’s really exciting to think about how this adjunctive therapy can help manage hypertension for many more people who've struggled to get their blood pressure under control in the past.”
The Heath Canada licence is based on clinical evidence backing the Symplicity blood pressure procedure - one of the most thoroughly tested cardiovascular interventional devices coming to market, backed by experience in more than 25,000 patients globally.* The procedure:
- …reduced patients’ office systolic blood pressure (OSBP) by more than 9 mmHg at three – six months, more than 18 mmHg (OSBP) at three years, and sustained blood pressure reductions at three years in more than 1,500 patients. 5-11
- ...demonstrated significant, safe, and durable blood pressure reductions in uncontrolled hypertension patients, independent of medications.5-7
- …showed very low rates of adverse events, making for a safe procedure.5-11
The Symplicity blood pressure procedure also demonstrated improved blood pressure control with significantly more time in treatment range after radiofrequency RDN when compared to sham through three years. 12
About the Symplicity blood pressure procedure
After sedation, the doctor makes a small incision and inserts a single thin tube (known as a catheter) into the artery leading to the kidney. Once the tube is in place, the doctor administers radiofrequency energy to the system to calm the excessive activity of the nerves connected to the kidney. The tube is then removed, leaving no implant behind.
Symplicity Spyral is approved for commercial use in more than 70 countries around the world.
Contacts:
Roxane Belanger
Public Relations, Canada
+1-416-553-0479
Ryan Weispfenning
Investor Relations
+1-763-505-4626
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies address 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary.
For more information, visit www.medtronic.ca and follow us on LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
* Data on file
1 Leung AA, Bushnik T, Hennessy D, et al. Risk factors for hypertension in Canada. Health Rep. 2019 Feb;30(2):3-13. Catalogue no. 82-003-X. PMID: 30747144.
2 Bundy JD, Mills KT, Chen J, et al. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. 2018 Jul 1;3(7):572-581.
3 U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Control Hypertension. Washington, DC: U.S. Department of Health and Human Services, Office of the Surgeon General; 2020.
4 Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387:957-67.
5Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. The Lancet. 2018 Jun 9;391(10137):2346-2355.2.
6Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomized, sham-controlled trial. The Lancet 2020; Published online March 29, 2020. DOI: 10.1016/S0140-6736(20)30554-7.3.
7Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. The Lancet. 2017;390:2160–2170.
8Schlaich et al, Kidney Week 2021
9 Bhatt DL, Vaduganathan M, Kandzari DE, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. The Lancet. Published online September 2022:S0140673622017871. doi:10.1016/S0140-6736(22)01787-1
10Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet 2022; 399: 1401–10.
11 Mahfoud F. ACC 2022
12 Kandzari D. EuroPCR 2022