
News Releases
Medtronic Receives Health Canada Licence for MiniMed™ 770g Insulin Pump System with Smartphone Connectivity for People with Type 1 Diabetes
New Smartphone Connected Hybrid Closed Loop System Will Be Available to Patients as Young as Two Years Old BRAMPTON, ON, Dec. 8, 2020 /CNW/ - Medtronic Canada ULC, a subsidiary of Medtronic plc...
New Smartphone Connected Hybrid Closed Loop System Will Be Available to Patients as Young as Two Years Old
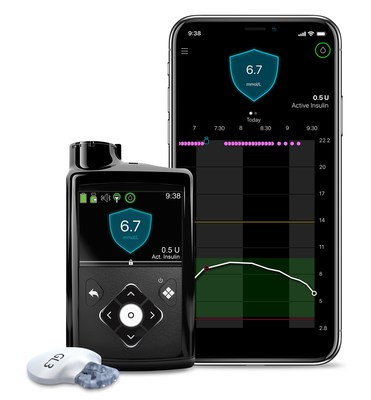
BRAMPTON, ON, Dec. 8, 2020 /CNW/ - Medtronic Canada ULC, a subsidiary of Medtronic plc (NYSE: MDT) – the world's largest medical technology, services, and solutions company – has received a licence from Health Canada for its MiniMed™ 770G insulin pump system. This is currently the only insulin pump system in Canada offering hybrid closed loop technology, with the added benefits of smartphone connectivity and an expanded age indication to children as young as two years.
This new system by Medtronic has the benefits of hybrid closed loop technology and has a high level of connectivity and convenience, making it easier to access and securely share real-time Continuous Glucose Monitoring (CGM) and pump data. The system:
- Lets caregivers and care partners see user data remotely on their smartphones.
- Sends proactive in-app alerts to both the user and their network when the user's glucose (sugar) levels are out of range.
- Allows data to be securely shared automatically with clinicians and educators - facilitating more effective telehealth visits and product trainings.
- Provides users with the ability to upgrade to future technology via software updates
- Gives users a choice in their diabetes management with flexibility to be used as a standalone pump as well as a CGM system with two options: suspend before a predicted low or automatic insulin adjustments every 5 minutes*
"We are very excited about this new insulin pump system because we believe these enhancements can help improve the safety and quality of life for people living with diabetes, and now for younger children too," says Laura Cameron, senior director of Diabetes at Medtronic Canada.
The growing body of clinical evidence on hybrid closed loop technology demonstrates the safety of the technology, as well as improved outcomes across adults, adolescents and younger children. A clinical study compared A1C and Time in Range of 151 children two to six years of age with those of 124 adolescents and adults over two weeks in Manual Mode and three months in SmartGuard™ Auto Mode (hybrid closed loop algorithm)1. The results showed positive outcomes in children comparable to those observed in older adolescents and adults with no episodes of severe hypoglycemia (low blood sugar), diabetic ketoacidosis (acidic blood), or serious device-related adverse events while in SmartGuard™ Auto Mode.
SmartGuard™ Auto Mode powers the hybrid closed loop technology in the insulin pump, allowing the round the clock system to continually adjust the amount of insulin delivered every five minutes, based on the user's needs. Automated delivery of background insulin regulates high and low glucose levels to maximize the length of time that blood glucose levels stay within the optimal target range. The MiniMed 770G system works with the Guardian™ Sensor (3)2, the MiniMed™ Mobile app (compatible with smartphones).
"With Canadians increasingly embracing virtual health visits and telehealth options, connectivity and convenience has never been more important," Cameron says, "this system offers access to the latest self-adjusting insulin pump technology, and increased connectivity to health care partners and caregivers – providing easier access to information and more peace of mind. Medtronic aims to help decrease the burden for people living with diabetes and MiniMed™ 770G technology will help enable this."
Medtronic Canada expects availability of the MiniMed 770G system in late winter 2021. New customers buying existing pumps today can upgrade to the MiniMed 770G system at no cost via Medtronic Canada's Next Tech Pathway program until the end of January 2021**.
*Some user interaction required.
**Terms and conditions apply.
About the Diabetes Group at Medtronic (www.medtronicdiabetes.ca)
Medtronic is working together with the global community to change the way people manage their diabetes. The company aims to transform diabetes care by expanding access, integrating care, and improving outcomes, so people living with diabetes can enjoy greater freedom and better health.
About Medtronic Canada ULC
Proud to serve Canadian healthcare for over 50 years, Medtronic Canada ULC (www.medtronic.ca), is a subsidiary of Medtronic plc, the world's largest medical technology, services, and solutions companies – alleviating pain, restoring health, and extending life for millions of people around the world. Serving physicians, hospitals, and patients across the country, Medtronic Canada ULC is headquartered in Brampton, Ontario, with regional offices in Montreal and Vancouver, and a Medtronic Resource Centre in Surrey, BC. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1 Salehi P, Roberts AJ, Kim GJ. Efficacy and Safety of Real-Life Usage of MiniMed 670G Automode in Children with Type 1 Diabetes Less than 7 Years Old. Diabetes Technol Ther. 2019;21(8):448-451. doi:10.1089/dia.2019.0123 |
2 The Guardian Sensor (3) is not intended to be used directly for making adjustments to insulin, but rather to provide an indication of when a finger stick may be required. All adjustments should be based on measurements obtained using a home glucose monitor and not on values provided by the Guardian Sensor (3). |
SOURCE Medtronic Canada ULC

