
News Releases
Medtronic Heart Pump is the First to Receive Health Canada Licence for Less-Invasive Implant Procedure
HeartWare™ HVAD™ System Also Receives Health Canada Authorization for Destination Therapy for Patients with Advanced Heart Failure Who Are Not Candidates for Heart Transplants BRAMPTON, ON,...
HeartWare™ HVAD™ System Also Receives Health Canada Authorization for Destination Therapy for Patients with Advanced Heart Failure Who Are Not Candidates for Heart Transplants
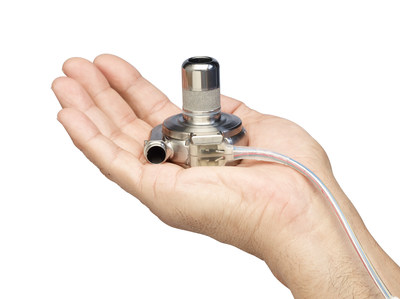
BRAMPTON, ON, Feb. 13, 2019 /CNW/ - Medtronic of Canada Ltd., a subsidiary of Medtronic plc (NYSE:MDT), today announced that Health Canada recently authorized a less-invasive procedure to implant its HeartWare™ Ventricular Assist Device (HVAD™) System, a left ventricular assist device (LVAD) for patients with advanced heart failure.

Approximately one million Canadians are currently living with heart failure, with a mean survival rate of just over two years.1 For patients with advanced heart failure, LVADs help the heart pump, and increase the amount of blood that flows through the body. They are typically implanted via median sternotomy, a surgical procedure in which a vertical incision is made down the middle of the chest, after which the sternum (or breastbone) is divided.
The HVAD System is the smallest commercially available LVAD2, and with this new licence from Health Canada, the HVAD System is the only LVAD authorized in Canada for implant via thoracotomy – a small, lateral, surgical incision between the patient's ribs on the left side of the chest. The thoracotomy approach may preserve the chest for future procedures, such as a heart transplant, and has been clinically shown to reduce the average length of recovery in hospital.3
Health Canada has authorized HVAD for implantation via thoracotomy based on the results of the LATERAL prospective clinical trial, in which 144 patients, with end-stage heart failure who were eligible for heart transplant, were enrolled at 26 centers in the U.S. and Canada. The primary endpoint of this trial demonstrated non-inferiority of the HVAD implanted in patients via thoracotomy, where survival at six months free from disabling stroke or device explant or exchange due to malfunction was achieved in 88.1 percent of patients.4 The LATERAL trial also showed a significant reduction in total length of hospital stay, from an average of 26.1 days down to 18 days.
"I have been implanting the HVAD system using the thoracotomy approach since 2010," says Dr. Anson Cheung, surgical director of the Cardiac Transplant Program of B.C., and principal investigator of the LATERAL trial. "In my experience, using this technique has resulted in better patient satisfaction, less need for blood product transfusion, shorter recovery and easier re-entry for subsequent transplantation. With the recent LATERAL trial, we have now shown that thoracotomy is a safe and effective technique for implanting the HVAD system resulting in shorter hospital stays and improved quality of life in our patients."
The HVAD System has also received authorization from Health Canada to modify the indications to include destination therapy (DT) in patients with advanced heart failure who are not candidates for heart transplants. Health Canada has authorized this indication in part due to results from the ENDURANCE and ENDURANCE Supplemental trials, which enrolled nearly 1,000 destination therapy patients. The data from these trials support the safety and effectiveness of the HeartWare HVAD System for patients with advanced, refractory left ventricular heart failure as a bridge to cardiac transplantation (BTT), or myocardial recovery, or as destination therapy in patients for whom subsequent transplantation is not planned.5
Worldwide, more than 17,000 patients have received the HVAD System since it was first approved in Europe in 2009.
About Medtronic Canada
Proud to celebrate 50 years in Canada in 2018, Medtronic of Canada Ltd. (www.medtronic.ca), headquartered in Brampton, Ontario, is a subsidiary of Medtronic plc, which is one of the world's largest medical technology, services, and solutions companies – alleviating pain, restoring health, and extending life for millions of people around the world. Medtronic of Canada Ltd. employs more than 1,100 people in Canada, serving physicians, hospitals, and patients across the country. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
_________________________ | |
1 | |
2 | HVAD System Instructions for Use. HeartWare, Inc., Framingham, MA, USA. 01/17. |
3 | Strueber M, et al. Impact of the Thoracotomy Implant Approach on Average Length of Stay and Rehospitalizations in the HVAD LATERAL Trial. Presented ISHLT 2018. http://abstractsonline.com/pp8/#!/4492/presentation/35403 |
4 | Emin A, et al. Quality of life of advanced chronic heart failure: medical care, mechanical circulatory support and transplantation. Eur J Cardiothorac Surg. 2016;50(2):269-73 |
5 | Rogers J, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017; 376:451-460 |

SOURCE Medtronic of Canada, Ltd.

